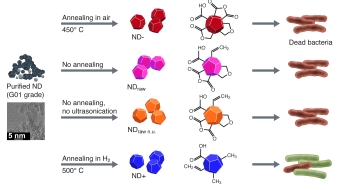
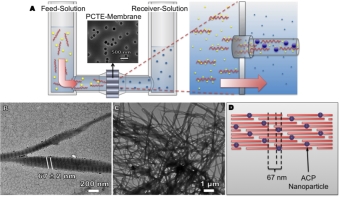
Nanodiamonds
Nanodiamonds are a fascinating material that only recently came into the spotlight despite being known for over sixty years. Recent breakthroughs in the last decade regarding processing, purifying, and dispersing nanodiamonds enabled widespread research on diamond nanoparticles and quickly showed the inherent potential of this newly rediscovered nanomaterial. Among the outstanding properties of the sp3 hybridized carbon nanoparticles are inherent fluorescence, excellent biocompatibility and ease of surface functionalization. Moreover, pure detonation nanodiamonds are easy to procure without being prohibitively expensive. Based on these features, nanodiamonds are starting to be intensely investigated as promising candidates for biomedical applications like drug delivery, nanoparticle-assisted diagnostics and imaging, or as implant coatings and reinforcements. Nanodiamonds have generally been considered biocompatible for a broad variety of eukaryotic cells.
In recent work,
we showed that, depending on their
surface composition, nanodiamonds
kill Gram-positive and -negative
bacteria rapidly and efficiently. We
investigated six different types of
nanodiamonds exhibiting diverse
oxygen-containing surface groups
that were created using standard
pretreatment methods for forming
nanodiamond dispersions. Our
experiments suggest that the
antibacterial activity of
nanodiamond is linked to the
presence of partially oxidized and
negatively charged surfaces,
specifically those containing acid
anhydride groups. Furthermore,
proteins were found to control the
bactericidal properties of
nanodiamonds by covering these
surface groups, which explains the
previously reported biocompatibility
of nanodiamonds. We are currently
starting to collaborate with several
institutes to further elucidate the
antibacterial surface properties of
nanodiamonds. These collaborations
will enable the detailed analysis of
the surface chemistry of
antibacterial nanodiamonds using
XPS, Raman and NMR, as well as
computational methods that model the
surface/biomolecule interactions of
nanodiamond particles. In another
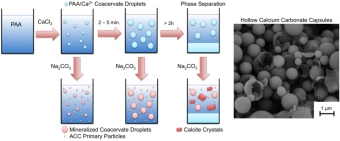
project, we are incorporating
nanodiamonds into bone replacement
materials based on hydroxyapatite in
order to provide these biomaterials
with antibacterial properties.